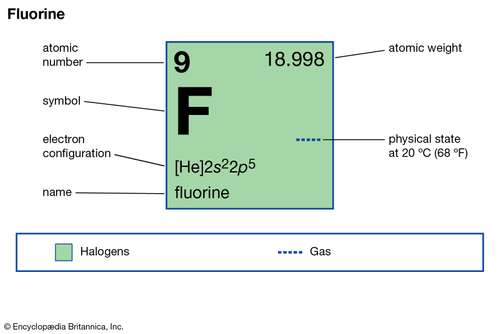
how many valence electrons does fluorine have
The power represents the total no. Fluorine symbol F is found in column.

Fluorine Valence Electrons Fluorine Valency F With Dot Diagram
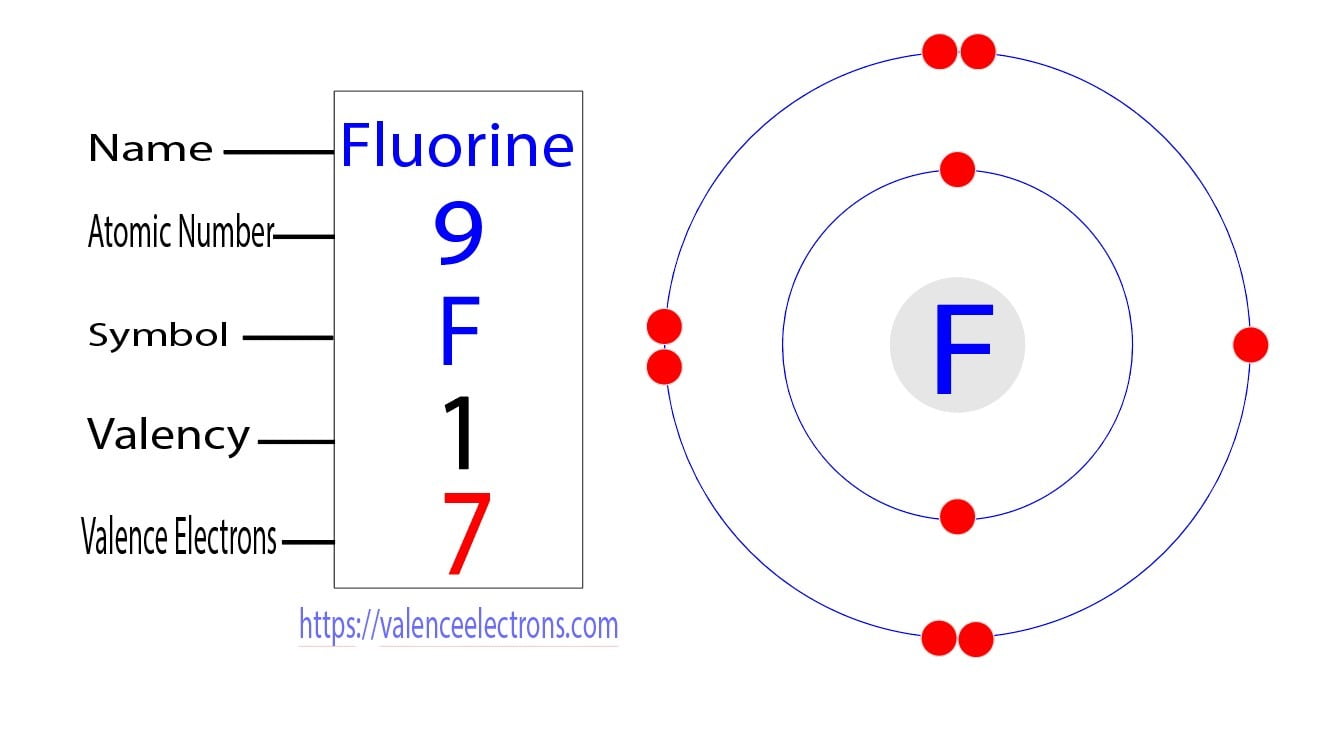
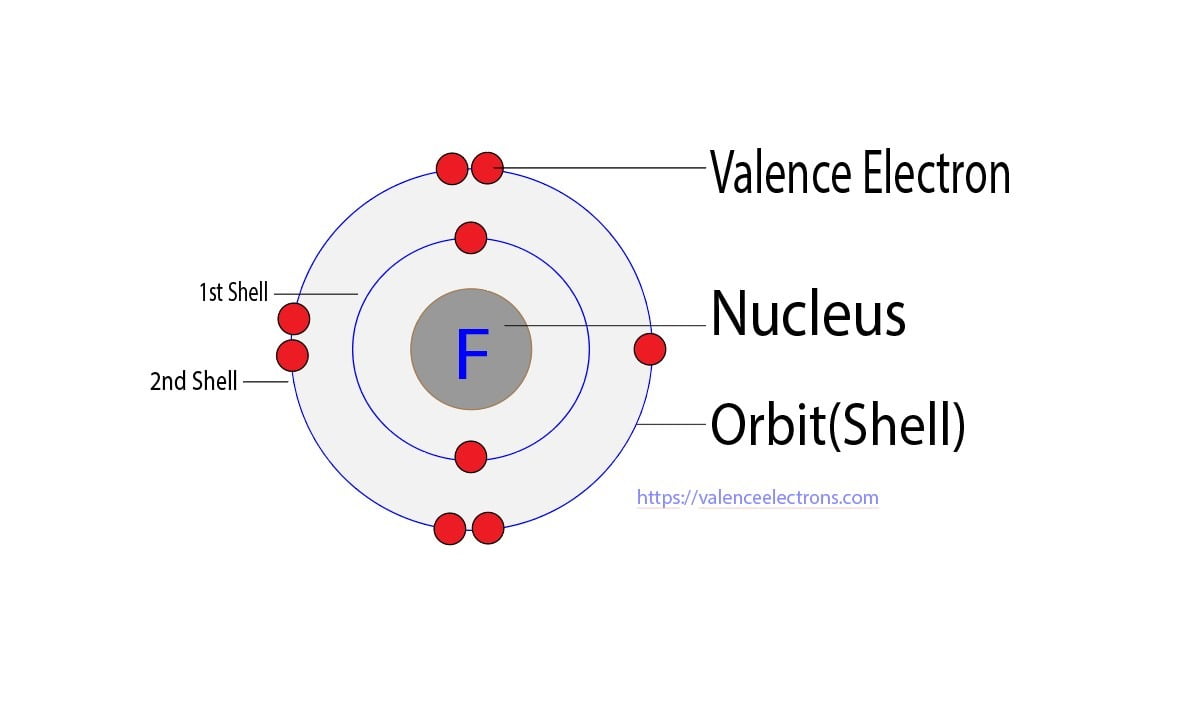
Trying on the image you possibly can see there are two electrons in shell one and 7 in shell two.
. After the electron configuration the last shell of the strontium atom has two electrons. We know the details about this. However the valence electrons of the transition elements may be in the inner orbital. 17 Why does sulphate have 4 Oxygens.
They are now stable as both fluorine have 8 valence electrons. After the electron configuration the last shell of the oxygen atom has six electrons. On the appropriate is the F2 molecule. Of electrons residing in that subshell.
Now both of the fluorine atoms have six electrons on their outermost shell. Fluorine has a total of 9 electrons. Is selected from the. Thus fluorine has seven valence electrons.
9 How many protons does scandium 45 have. Therefore the valence electrons of fluorineF are seven. Play to see the electrons orbiting the nucleus of each atom. The atom of fluorine prefers to gain an electron to complete its last orbit.
The outermost electrons in each atom are called. Fluorine represented by F is an element with atomic number 9 meaning that it has 9 protons in its nucleus. On the right is the F2 molecule. Select a substance.
How many valence electrons does fluorine have based on its location on the periodic table. 10 How do you find the scandium electron configuration. Number of valence electron in fluorine element 2 5 7. 15 How many sulfur atoms would combine together to form a stable molecule.
16 Does sulfur form ionic bonds. A fluorine atom has 7 valence electrons. Click Pause. The elements that have 1 2 or three electrons in the last shell donate the electrons in the last shell during bond.
As Fluorine F has 7 valence electrons it has a tendency to accept another electron and complete an octet 8 electrons. Fluorine belongs to group 7 in the periodic table. Fluorine has 7 valence electrons. Herein how many electrons does a fluorine atom have in its second shell.
Thus fluorine has seven valence electrons. How many valence electrons does oxygen ionO 2- have. In this case the valence electrons of oxygen are 6. How many valence electrons does strontium ionSr 2 have.
The electron configuration of fluorine shows that the valence electrons of fluorine are seven. See full answer below. That means there are 9 electrons in a fluorine atom. Electron configuration of F using Aufbaus Principle F 1 s 2 2 s 2 2 p 5 where the no.
Beside above whats the complete variety of electrons in a fluorine molecule f2. Which means there are 9 electrons in a fluorine atom. How many valence electrons does fluorine have. 13 Does indium have 3 valence.
On the left is a fluorine atom with seven valence electrons. 14 Does sulfur form ionic or covalent bonds. Fluorine has 7 valence electrons however Fluoride F- is an anion with a negative charge giving it one more electron bumping the number of valence electrons to 8. The valence electrons are in the last shell of the element.
The beryllium atom donates its valence electrons to the fluorine atom and the fluorine atom receives those electrons. Group 7 elements are known as halogens. Fluorine is halogen and is the most electronegative element among the halogens. The electron configuration of helium shows that there are a total of two electrons in the last shell orbit of.
On the left is a fluorine atom with seven valence electrons. Neon has 10 electrons --- 2 in the first shell and 8 in the second shell so eight valence electrons. Click to see full answer. Drag an electron from the left atom to the right atom.
Looking at the picture you can see there are two electrons in shell one and seven in shell two. Fluorine has 9 electrons--- 2 in the first shell and 7 in the second shell so seven valence electrons. The electron configuration of fluorineF shows that the last shell of fluorine has seven 2s 2 2p 5 electrons. How many valence electrons does each fluorine atom have.
8 Does scandium have 2 or 3 valence electrons. Fluorine F has 7 valence electrons. Theres currently two electrons one pair orbiting around the oval shell that connects the two fluorine atoms. The diatomic fluorine molecule F2 contains a.
The atomic number of fluorine is 9 and its electronic configuration is He 2s2 2p5. 11 Can sulfur have more than 8 valence electrons. 12 How many bonds can Cl make. 12 What is the atomic number.
In front of the letter represents the principle quantum shell. The letter represents the subshell. The fluorine atom has seven valence electrons. The total number of electrons present in the valence shell of an atom is called valence electrons and there are a total of seven electrons present in the valence shell of fluorine 2s22p5.
The total number of electrons present in the valence shell of an atom is called valence electrons and there are a total of seven electrons present in the valence shell of fluorine 2s22p5. Fluorine forms a negative ion by gaining an. 13 How many electrons does sulfur have. We know the details about this.
Fluorine is the 9th element of the periodic table having electronic configuration of. 11 What is an example of a valence electron. 18 Why can sulfur exceed the octet. Also Know what is the total number of electrons in a fluorine molecule f2.
6 How many valence electrons does fluorine have. Hence the given element is non-metal and the number of valence electrons are 7. Seven valence electrons For example fluorine has seven valence electrons so it is most likely to gain one electron to form an ion with a 1- charge. In this case the valence of strontium is 2.
7 What family has 8 valence electrons. After arranging the electrons it is seen that the last shell of the oxygen atom has six electrons. This element will gain 1 electron to form ion.

How Many Valence Electrons Does Fluorine F Have Valency Of Fluorine

How Many Valence Electrons Are In Fluorine Quora

How Many Valence Electrons Are In Fluorine Quora
Posting Komentar untuk "how many valence electrons does fluorine have"